
These reporter enzymes produce signals by reacting with substrates to cause color changes or produce light changes. In order to produce an observable signal, antibodies are often linked, through their Fc region, to a reporter enzyme, such as alkaline phosphatase or horseradish peroxidase. In immunoblotting, this region is mainly utilized as the epitope for a secondary antibody – an antibody which recognizes the first antibody that has bound to the protein you’re trying to detect. In addition to Fab fragments, antibodies contain an Fc region, which is specific to the animal that produced the antibody. This is important because many antibodies only recognize conformational epitopes, which means that they recognize proteins in their native 3D state only. Monoclonal antibodies that recognize a linear epitope are preferred as that ensures the epitope can be found on a denatured, or linearized, protein.
#Procedure of western blot series#
In contrast, polyclonal antibodies are a series of different antibodies that target many epitopes on the same antigen – or protein for which an antibody has specificity. Monoclonal antibodies are antibodies that recognize a single epitope and are the preferred antibody type used for immunoblotting due to their specificity. The Fab region defines the specific epitope, or specific portion of a protein, to which an antibody will bind. Antibodies are large Y-shaped proteins that contain two fragments, also known as Fab regions, which bind to other proteins. Immunoblotting uses antibodies to “probe” the membrane for specific proteins. During the transfer, an electric field is used, to move the proteins through the gel, where they become trapped on a membrane due to charged and hydrophobic interactions. The electroblotting sandwich consists of the gel and a specialized membrane, sandwiched between two pieces of filter paper. Before these stages are attempted, SDS-PAGE, in which denatured proteins are separated by size in a polyacrylamide gel, must be performed.Įlectroblotting, is also known as the Western “transfer” and requires a transfer cassette for holding together the “sandwich” as well as an apparatus for transferring protein from an acrylimide gel to a thin membrane. There are 3 principal stages of this technique that are essential for a quality outcome: Electroblotting, Immunoblotting, and Detection. The broad applications of this technique are described through several examples including the detection of protein-protein interactions and identification of individual proteins within protein complexes. The steps involved with western transfer such as the assembly of the transfer sandwich and transfer conditions are discussed in detail as well as the theory behind antibody binding and detection of those antibodies. This video-article presents an overview of the western blot technique by describing western transfer, the use of antibody detection, and image analysis. These signals can then be imaged and quantified using a process called densitometry.

Enzymes can be attached to the end of an antibody and react with substrates to produce changes in color or light. The detection of antibodies takes place using reporter systems which includes the use of enzymes. Immunoblotting uses antibody-protein and antibody-antibody binding through specific recognition sites, providing the high specificity required for identifying a single protein. Next, the membranes are probed with antibodies in a process called immunboblotting.

Following separation by a technique known as sodium dodecyl sulfate polyacrylamide gel electrophoresis, or SDS-PAGE, western transfer is used to move proteins from a polyacrylamide gel onto a piece of membrane which traps the proteins in their respective locations.
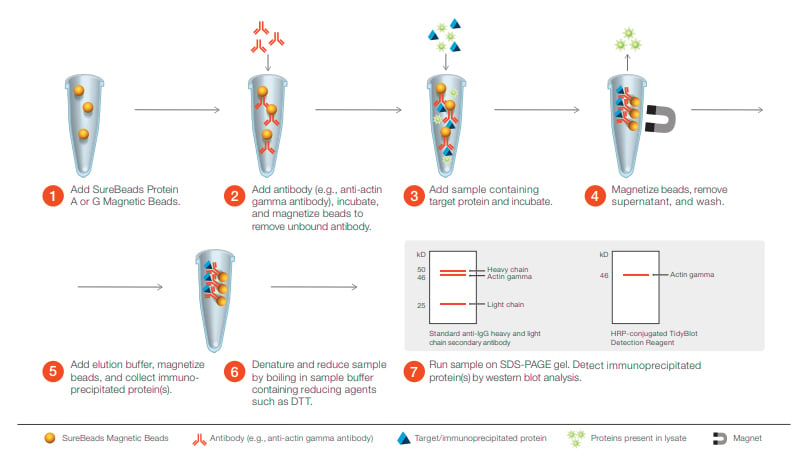
Western Blotting is used to identify the presence of specific proteins in electrophoretically separated samples.


 0 kommentar(er)
0 kommentar(er)
